Moderna’s Covid-19 vaccine shows nearly 95% protection
BI Report || BusinessInsider

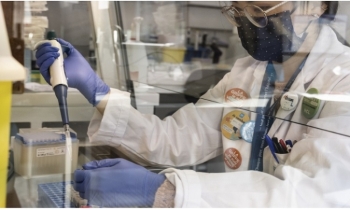
A new vaccine that produced in fighting against the Covid-19 is nearly 95 percent effective, early data from US company Moderna shows today.
The results come hot on the heels of similar results from Pfizer, and add to growing confidence that vaccines can help ending the coronavirus pandemic across the world.
Both the companies used a highly innovative and experimental approach to designing their vaccines in fighting the pandemic.
Moderna says it is a “great day” and they plan to apply for approval to use the vaccine in the next few weeks, BBC news told.
Moderna, in a press release in its official website, said the company involved 30,000 people in the US with half being given two doses of the vaccine, four weeks apart. The rest had dummy injections.
The primary endpoint of the Phase 3 COVE study is based on the analysis of Covid-19 cases confirmed and adjudicated starting two weeks following the second dose of vaccine.
Only five of the Covid-19 cases were in people given the vaccine, 90 were in those given the dummy treatment. The company estimates of vaccine efficacy of 94.5 percent, the press release read.
“This is a pivotal moment in the development of our Covid-19 vaccine candidate. Since early January, we have chased this virus with the intent to protect as many people around the world as possible. All along, we have known that each day matters.
This positive interim analysis from our Phase 3 study has given us the first clinical validation that our vaccine can prevent Covid-19 disease, including severe disease,” Stéphane Bancel, Chief Executive Officer of Moderna said in the press release.